How QuikVue and VisuDoc Support Ocular Toxicity Monitoring in Drug Development
In pharmaceutical R&D, every promising therapy must clear a critical hurdle: safety. For drugs that are either delivered directly to the eye or have systemic effects that may impact ocular tissues, monitoring for eye toxicity is both a regulatory and ethical necessity.
While ocular safety assessments have traditionally relied on large, fixed slit-lamp camera systems, the development landscape is changing. Portable imaging technologies like QuikVue, combined with systematic documentation tools such as VisuDoc, are making it easier than ever to capture, organize, and compare high-quality images of the cornea and ocular surface — even across multiple trial sites.
Why Ocular Toxicity Monitoring Matters
The cornea and ocular surface are delicate, highly innervated tissues that can be affected by:
Topical ophthalmic drugs (e.g.,anti-glaucoma agents, anti-infectives)
Systemic therapies (e.g., oncology treatments, immunosuppressants, antivirals)
Preservatives and excipients in formulations
Potential side effects include:
Corneal epitheliopathy (punctate keratitis, epithelial erosions)
Conjunctival hyperemia and inflammation
Tear film instability and dry eye symptoms
Limbal vascularization or pannus formation
Regulatory bodies like the FDA and EMA require consistent, documented monitoring of such changes during trials — particularly in ophthalmology, oncology, rheumatology, and infectious disease drug development.
QuikVue+ VisuDoc: A Practical Ocular Safety Combination
1. Documenting Visible Changes
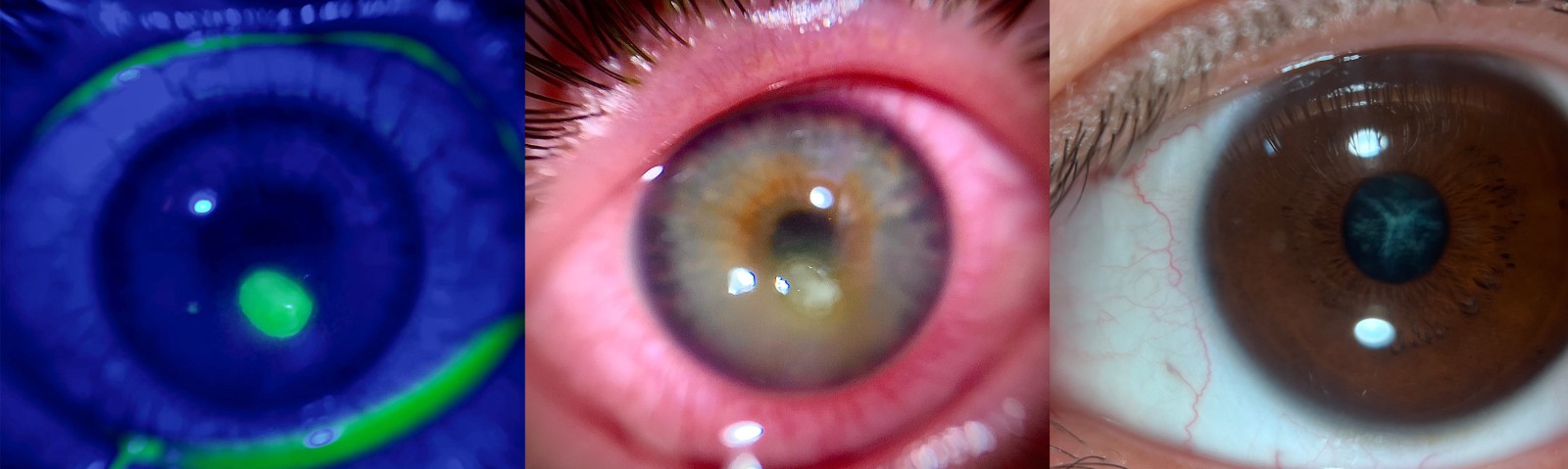
With 10–20× magnification and diffuse illumination, QuikVue is ideal for capturing gross, clinically visible changes on the ocular surface and cornea.
These include:
Significant epithelial erosions or defects
Conjunctival redness and swelling
Obvious corneal opacity
VisuDoc complements this by:
Storing images securely in patient profiles in local server
Organizing them chronologically
Enabling side-by-side comparisons for monitoring progression or recovery
2. Objective Evidence for Safety Reporting
Consistent, high-quality images paired with structured records in VisuDoc help trial teams:
Document ocular adverse events with photographic proof
Support safety narratives in case reports and regulatory submissions
Reduce reliance on purely narrative or subjective grading
3. Multi-site Consistency
Large slit-lamp imaging setups differ from site to site, which can affect trial image comparability.
With QuikVue:
Every site can use the same image device model
We recommend using the same smartphone model across sites for hardware consistency and uniform image output
Portable design allows deployment to both major and smaller research centers
Advantages for Pharmaceutical Sponsors
Cost-effective: More sites equipped for less than a single slit-lamp camera.
Fast capture: Minimizes patient chair time.
Minimal training required: Even non-specialist staff can reliably document findings.
Regulatory support: Helps meet ICH, FDA, and EMA expectations for documenting visible ocular changes.
Integrated workflow: QuikVue for imaging + VisuDoc for structured, secure management.
Example Use Cases
Oncology trials – Recording conjunctival inflammation from systemic agents.
Glaucoma drug studies – Documenting preservative-related redness or epithelial damage visible at clinical scale.
Antiviral trials – Monitoring corneal clarity and conjunctival condition.
Contact lens solution evaluations – Capturing ocular surface compatibility issues.
Positioning in the Drug Development Workflow
"QuikVue and VisuDoc provide a practical, scalable way to document and manage visible ocular surface changes during drug development. By combining portable imaging with structured documentation, sponsors can monitor patient safety consistently across multiple sites — without slowing trial timelines."
While QuikVue isn't designed for microscopic or subclinical changes, it's a reliable, accessible tool for capturing and tracking visible ocular surface and corneal findings that matter in later-stage toxicity monitoring and regulatory reporting. Paired with VisuDoc, it delivers a complete workflow from image capture to comparative analysis.
QUIKVUE IN OCULAR TOXICITY MONITORING | ||
| Category | Strengths – Where QuikVue Excels | Limitations – What QuikVue Is Not Designed For |
| Magnification & Illumination | 10X–20X magnification with diffuse illumination. Ideal for gross,clinically visible changes. | Cannot resolve microscopic or subclinical epithelial changes |
| Detectable Changes | Corneal opacity, significant epithelial defects,conjunctival hyperemia, limbal vascularization. | Cannot detect early micro-abrasions, subtle tear film disruptions, or cellular-level changes. |
| Deployment in Trials | Portable, quick setup, easy to deploy across multiple sites. | Not a replacement for high-end slit-lamp cameras in trials requiring fine structural analysis. |
| Consistency Across Sites | Same device model across all sites; Recommend same smartphone model for hardware consistency. | Image quality still dependent on ambient conditions and user technique. |
| Workflow Integration | Works seamlessly with VisuDoc for systematic documentation, side-by-side comparison, and storage. | Requires separate staining/diagnostic procedures for certain ocular surface conditions. |
| Cost & Training | Low cost, minimal training needed, fast capture | Not suitable for highly specialized imaging (confocal, specular microscopy) |